Theory of Light and Color

2. Light as Electromagnetic Waves
Last time, we explained that light is one form of electromagnetic waves, and the concept of light, which is based on human sense, was historically expanded to the broader concept of ultraviolet, visible, and infrared as physical energy of the electromagnetic waves.
This time, we would like to talk about the basic properties of electromagnetic waves and the common properties of light.
What Makes Up Electromagnetic Waves?
Electromagnetic waves are a physical phenomenon in which energy vibrates between an electric field and a magnetic field. It includes wavelengths that are much shorter than light such as γ (gamma) rays and X-rays, as well as much longer wavelengths including microwaves and radio waves.
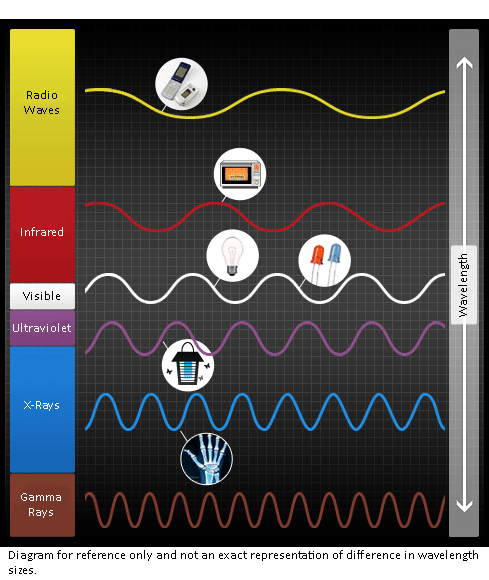
These electromagnetic waves have various uses in everyday life.
・Radio waves: short-, medium-, and long-wave types are used for radio and TV broadcasting
・Microwaves: used in microwave ovens
・Light: ultraviolet, visible light, and infrared
・X-rays: used for medical imaging
Electromagnetic Waves as Waves and Particles
All electromagnetic waves are composed of photons. A photon is the smallest discrete amount or “quantum” of electromagnetic radiation. It is the basic unit of all light.
Electromagnetic waves have both wave-like properties and particle-like properties, illustrated in the examples below. This property is one of the central principles of modern quantum mechanics.
A pebble thrown into a pond creates ripples. An object floating on the water vibrates up and down with the ripples but stays in place (unless moved by an outside force like wind). The wave energy propagates to the surroundings along with the ripples.
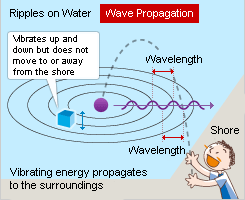
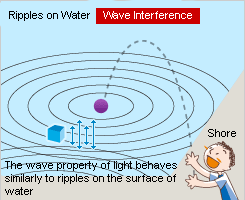
The ripples on the water are reflected in the opposite direction after hitting the shore. The incoming waves and the returning waves overlap, which can strengthen or weaken each other. This is called the wave interference phenomenon. The object floating on the water irregularly vibrates up and down based on the overlapping of the waves.
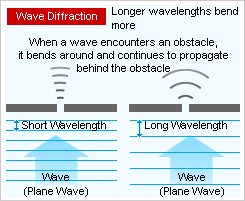
Another phenomenon called wave diffraction occurs when waves encounter an obstacle and bend around it. The longer the wavelength, the greater the bend around the obstacle.
Electromagnetic waves behave similarly to ripples due to their wave nature. ≪1≫
For example, electromagnetic waves exhibit both the wave diffraction and interference phenomenon, which becomes more prominent as the wavelength gets longer. The radio and television waves sent from broadcasting stations can be received even in the shadows of buildings or indoors because their long wavelength causes greater diffraction.
As wavelength gets shorter, the wave nature becomes less prominent, and the electromagnetic waves will travel straight ahead with little diffraction, behaving more like a particle. ≪2≫ This can be seen with visible light. Although it has both wave and particle properties, its shorter wavelength appears to move linearly and thus causes the appearance of shadows. However, its diffraction effect is visible when using certain experimental devices.
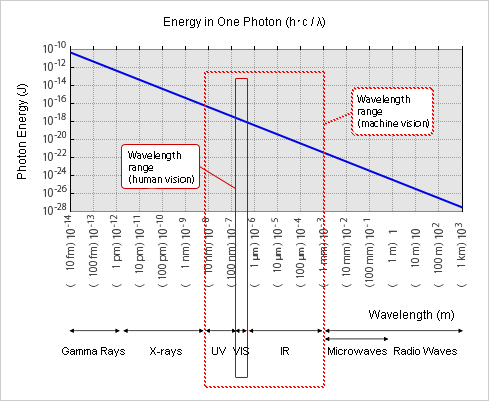
Shorter Wavelength Equals Greater Energy
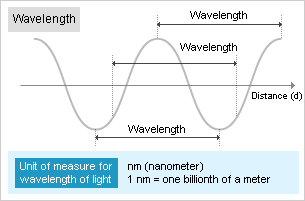
The energy of photons is inversely proportional to the wavelength. Photons have higher energy as the wavelength becomes shorter. ≪3≫
Each photon carries energy and collides with various objects, including the human eye and skin. High-energy photons can change or destroy the molecular structure of an object. This is why shorter wavelengths like γ (gamma) rays and X-rays are hazardous and cause severe damage to the living tissues of humans and animals. In the case of non-living objects such as paper or cloth, for example, it may cause deterioration or discoloration.
One photon’s energy is extremely small for humans to perceive, but innumerable photons gathered together (in the visible wavelength range) allow humans to sense "brightness."
The Environment and Electromagnetic Waves
The sun emits various types of electromagnetic waves. Harmful ones that have wavelengths shorter than 280 nm (γ (gamma) rays, X-rays, UV-C, etc.) do not reach the Earth’s surface because they are mostly absorbed by the atmospheric layer above the earth. One of the reasons astronauts wear spacesuits outside the spacecraft is to protect their bodies from the harmful electromagnetic waves like gamma rays, X-rays, and UV rays above the atmosphere.
About 0.5% of UV-B (280-315 nm) and 5% of UV-A (315-380 nm) are said to reach the surface depending on the season and weather. One exception is the ozone hole above the Antarctic region. It is regarded as a serious environmental concern because ultraviolet (UV), which should be absorbed in the ozone layer, passes through the ozone hole and reaches the Earth.
On the other hand, most visible and infrared light reaches the Earth after being partially absorbed by atmospheric moisture. Life on Earth can exist thanks to solar energy from the visible and infrared range while the atmosphere blocks shorter wavelengths that harm the body.
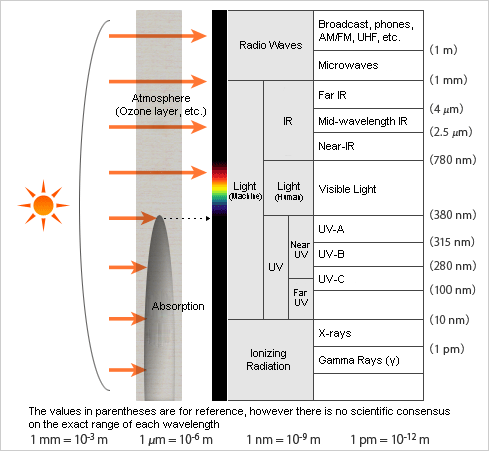
Humans and the Electromagnetic Spectrum
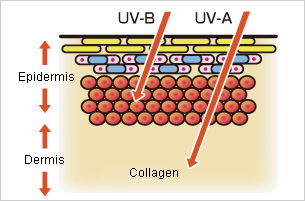
Electromagnetic waves with wavelengths in the UV-C range are extremely damaging to living organisms. Furthermore, UV-B and UV-A that partially reach Earth are not completely safe.
UV-B is the main cause of damage that results from extended exposure to strong direct sunlight, including sunburns, skin cancer, and even damage to DNA. It also causes a type of eye inflammation called snow blindness, where UV rays reflected off snow give eyes sunburn. Although UV-A is less dangerous than UV-B, it can reach deep inside the skin, denaturing collagen that makes up the skin’s tissues and promoting skin aging like spots and freckles.
Even wavelengths longer than UV can cause damage. An intense blue light or overexposure to it carries the risk of damaging your retina when directly looking at it.
X-ray imaging usually does not cause any problem for humans since the X-ray irradiates only for a short time, but there is a high risk for X-ray technicians who are repeatedly exposed, which is why they take the image from a separate room.
Sunlight can turn white paper yellow over time because the photon energy of shorter wavelengths damages the paper’s molecular structure. The damage makes it harder for the paper to reflect shorter wavelengths in the visible light range, thus changing its color.
Electromagnetic waves with long wavelengths (infrared or radio waves) have less photon energy, which makes them less harmful to the body, but that does not mean there is no danger at all. For example, glassworkers can develop cataracts over time due to infrared radiation from extreme heating of the glass. All of the above examples of negative effects on the living body and objects are related to the wavelength dependence and intensity of electromagnetic energy.
Light is also a type of electromagnetic wave. When using light in machine vision, wavelength and the corresponding photon energy are important factors to consider. Different light phenomena occur depending on the wavelength, which makes it possible to highlight and thus capture the target information. You should avoid looking directly into the light source as much as possible when using the light, especially when using ultraviolet (UV) and short-wavelength visible light (violet, blue, etc.).
Comment
≪1≫ Electromagnetic wave
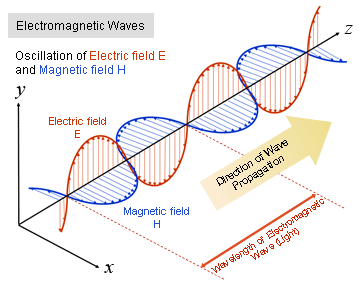
Electromagnetic waves are a physical phenomenon in which energy vibrates between an electric field and a magnetic field.
An electric field is a property of space that surrounds an electric charge and exerts force on other charges in the field. There are two types of electric charge: positive (+) and negative (-). Like charges repel each other and opposite charges attract each other.
A magnetic field is a property of space that exerts force on magnetic poles (north pole and south pole of a magnet).
The electric and the magnetic field are closely related to each other.
When the magnetic field changes, it exerts force on electric charges to move them (current flow), and when current flows, it changes the magnetic field (electromagnetic induction). Synchronized oscillations of electric and magnetic fields are created by the interaction of the two, and electromagnetic energy propagates as a transverse wave in space.
The oscillations of the electric field H and the magnetic field E are perpendicular to each other and perpendicular to the direction of energy and wave propagation Z.
≪2≫ Light generation mechanism
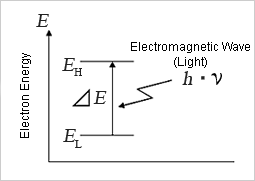
Everything in nature is made up of atoms, and atoms are made up of nuclei and electrons as shown on the right. The electron orbits the nucleus in multiple energy states. This is only an approximate model as the electron orbits are probabilistic in nature.
When the energy state of the electron changes from high energy (EH) to low energy (EL), the energy difference (ΔE = EH-EL) will be released from the atom as wave energy. This is light itself (electromagnetic waves). The photon is the smallest unit (quantum) of the wave energy that forms electromagnetic waves.
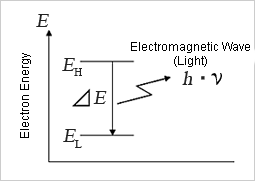
≪3≫ Photon energy and wavelength
Photon energy is proportional to the frequency ν (read as “new”), so the emitted wave energy ΔE [J (Joules)] is
ΔE = EH - EL = h ・ ν
(This proportional constant h is called Planck's constant. h ≈ 6.6 × 10-34 [J x s (joule seconds)])
Furthermore, if the wavelength of light is λ [m] (read as “lambda”), and the frequency is ν [1/s], the traveling speed c [m/s] is
c = ν ・ λ
(c is a constant, c = 3 × 108 [m / s] = 300,000 km / s in a vacuum)
Thus,
ΔE = EH - EL = h ・ ν = h ・ c / λ
The energy and momentum of a photon depends on its frequency (ν) or inversely, its wavelength (λ)
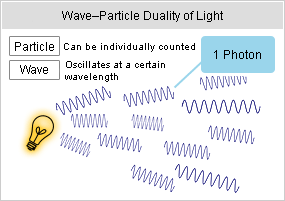
The above focuses on one electron. When such a phenomenon occurs with a large number of atoms, numerous quanta (photons) of electromagnetic energy will be released one after another by the transition of each electron, and vibrate with its wavelength corresponding to each energy as it moves through space.
From the figure shown on the right, you can roughly understand that light behaves like a wave and a particle.
For example, an incandescent bulb works based on the principle that light is emitted when the energy level of electrons in numerous atoms changes from a high level EH to a lower level EL after being excited to the high level by the injection of electrical energy.
Conversely, when light with a certain wavelength (frequency) interacts with atoms, the light gives its energy to electrons if certain conditions are met.
The energy imparted by the light causes electrons to move to a higher level. This is called excitation of the energy state of electrons due to the light. The phenomenon where light energy is transferred to electrons is called absorption of light. For example, when putting a white paper and a black paper under direct sunlight, the black one gets higher in temperature because it absorbs more light energy.